Caustic soda tests measure the purity level of the caustic soda. Caustic soda quality must be controlled because it naturally contains some impurity.
It may have small amounts of other chemical compounds such as sodium carbonate, sodium sulfate and iron. You may find them in the caustic soda specifications table. Would you want to learn how they are measured?
This article provide a simple and brief explanation of some of the most important caustic soda tests.
Total Alkalinity of Caustic Soda
Caustic soda is alkaline due to the presence of sodium hydroxide (NaOH) and sodium carbonate (Na2CO3).
The total alkalinity of caustic soda is determined by titration with a hydrochloric acid (HCL) standard solution and methyl orange as a color indicator.
Add some acid drop by drop to the caustic soda solution until it changes the color. This test tells you how much HCL you need to neutralize a solution of caustic soda with a certain concentration.
Use the following formula to find out how much the alkalinity of caustic soda is:
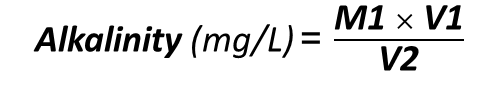
M1= HCl Concentration (mg/L)
V1= Used HCl Volume (L)
V2= Volume of Caustic Soda Solution (L)
Sodium Hydroxide Content of Caustic Soda
Caustic soda is commercial sodium hydroxide, which may include impurities like sodium carbonate and sulfates. So it’s important to know exactly how much sodium hydroxide (NaOH) content is in caustic soda.
First, add barium chloride (BaCl2) to the caustic soda solution. This makes sodium carbonate (Na2CO3) precipitate. Next, titrate the remaining solution using a standard hydrochloric acid (HCL) solution and a few drops of methyl orange.
The following formulas are used to determine the sodium hydroxide (NaOH) content in caustic soda:

M1= HCl Concentration (mg/L)
V1= Used HCl Volume (L)
V2= Volume of Caustic Soda and BaCl2 solution (L)
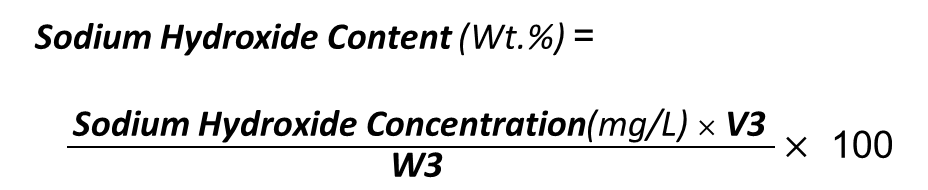
V3=Volume of Caustic Soda Solution (L)
M3 = First Amount of Caustic Soda (mg)
Sodium Carbonate Content of Caustic Soda
Caustic soda has the ability to absorb and react with CO2 from the air, resulting in the generation of sodium carbonate (Na2CO3). This reaction decreases the purity of caustic soda. This is why we should keep it in a completely closed package.
There are several ways to understand how much sodium carbonate is in caustic soda. But the easiest way is to use the amounts of total alkalinity and sodium hydroxide in caustic soda that were explained in the last part.
The following formula should be used in this method:
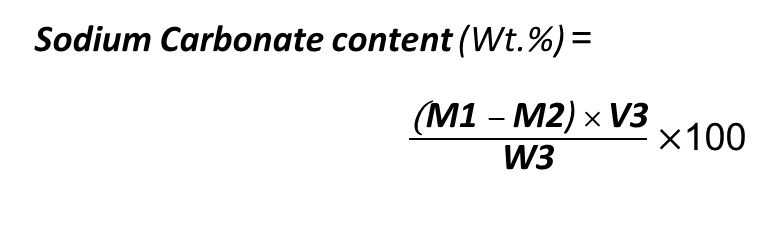
M1= Alkalinity (mg/L)
M2= Sodium Hydroxide Concentration(mg/L)
V3= Volume of Caustic Soda solution (L)
W3= First Amount of Caustic Soda (mg)
Sulfate Content of Caustic Soda
Sulfate is present in caustic soda in the form of sodium sulfate (NaSO4). For measuring the content of sulfates, we need HCl solution and BaCl2. First, a solution of caustic soda is neutralized by HCl. Then, add an excess amount of HCl and boil the mixture. In this process, some solid materials are produced, which should be filtered.
Now the remaining solution is free of all carbonates and chlorides. Add a certain amount of BaCl2 to it, and measure the weight of the precipitate that is produced.
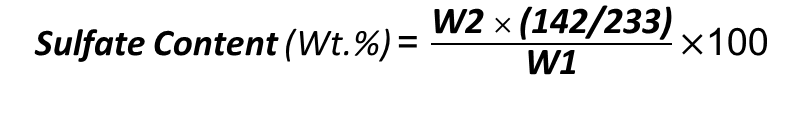
W1= First Amount of Caustic Soda (mg)
W2= Weight of precipitate(mg)
Iron Content of Caustic Soda
Iron can result from contamination during storage or transport of the caustic soda. In this section we explain how iron content in caustic soda is measured briefly.
At the first a caustic soda solution is prepared and neutralized with HCl solution. Then an excess amount of HCl will be added to have an acidic solution. The pH can be checked by a pH test ribbon.
Generally, iron exists in caustic soda in two forms: ferric and ferrous. Hydrogen peroxide is used to convert any ferrous ion that may be present in the caustic soda solution to its ferric state. Because in this test we just can measure the ferric iron content.
In the final step some potassium thiocyanate solution (KSCN) is added. Ferric iron in an acidic medium reacts with thiocyanate ions to produce a red color complex. The KSCN only reacts with ferric ions, that is the reason for adding Hydrogen peroxide in previous steps.
The intensity of the color is related to the amount of iron present. By measuring the color intensity with a spectrophotometer, the concentration of iron in a sample of caustic soda can be determined.
If you are interested in knowing the accepting limit for each property, check out the caustic soda specifications table. Leave a comment with any questions about caustic soda.








