Caustic soda can be made through several processes as a liquid in different concentrations.
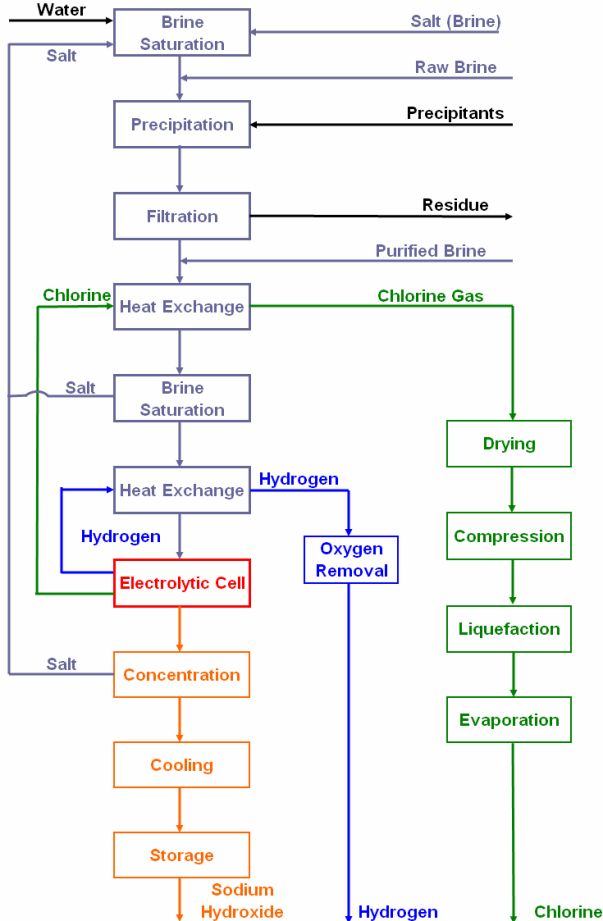
Electrolysis, which means passing an electrical current through brine (salt dissolved in water), is a method that has been used for 120 years to produce caustic soda.
It is performed in different cells included: membrane cell process, mercury cell process, and diaphragm cell process.
In the following, we explain more details about these methods to produce caustic soda.
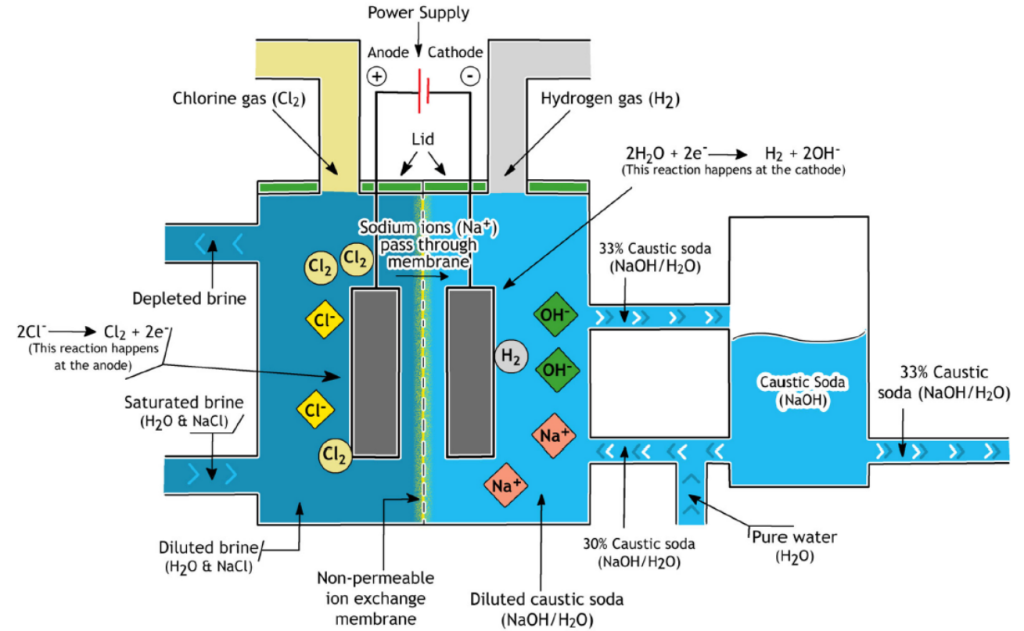
Membrane Cell Process
The membrane cell process comprises almost 85% of the installed capacity in Europe and has the dominant share of sodium hydroxide production among the other two methods.
For producing caustic soda, we require salt and electricity to make the reaction happen.
Membrane cell is the cheapest sodium hydroxide production method, considering energy and capital cost. What makes these kinds of cells important is that they do not cause environmental concerns.
As discussed in the previous caustic soda article, lye is usually sold in the market, with 50% saturation. However, the lye obtained from the membrane cell process is just 30% rich.
This is because some fraction of caustic soda produced in the cathode section is removed and the other fraction is diluted to obtain a 30% lye. This diluted lye is then returned to the cycle( cathode section) to keep the cell operating.
The caustic soda obtained with this method has about 13% concentration which further increases to 50% due to evaporating the 13% solution.
The caustic soda produced with this method has a growing acceptance in rayon fiber production.
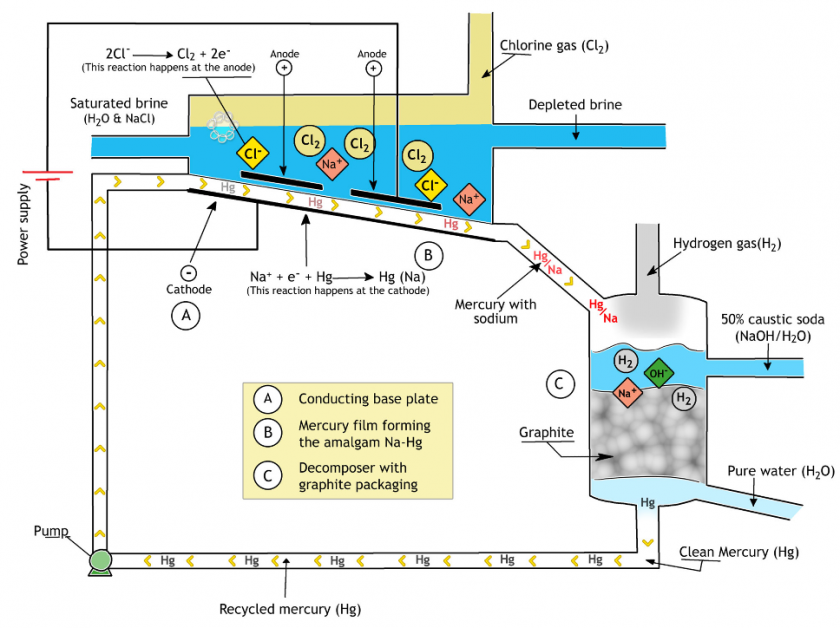
Mercury Cell Process
However, the present mercury in the system limits the applications of this method since mercury is a hazardous material for the environment.
In the mercury cell method, mercury plays the role of cathode and the produced sodium will react with water to produce sodium hydroxide or caustic soda liquid (lye).
The Mercury cell method is rarely used these days since it is not a safe technology to work with.
In this process, saturated brine water flows downward in a steel passage.
This passage is about 15 meters long and 1 meter wide.
The inner area is covered with a mercury film, also streaming downward (where the mercury layer remains down due to density difference).
This mercury layer acts as the cathode, and the titanium plates act as the anode with a direct current being applied to these poles. The result is the release of chlorine at the anode and sodium ions at the mercury film surface which is the cathode.
This released sodium ions mixes with mercury and streams out of the cell without reacting with water or chlorine gas.
Regarding the previous discussion, the mercury cell method has two products which are hot, wet, and corrosive chlorine gas and sodium amalgam.
The soda cell or decomposer is where the mercury is separated from the amalgam stream. The decomposer is made of a cylindrical steel chamber that is loaded with graphite balls. These balls help to extend the contact surface and hence, increase the reaction speed.
The inlet streams are sodium amalgam and pure water. In the presence of graphite, the streams react, and the results are sodium hydroxide and hydrogen gas. Mercury separates in the process and can be reused in the electrolyte cell.
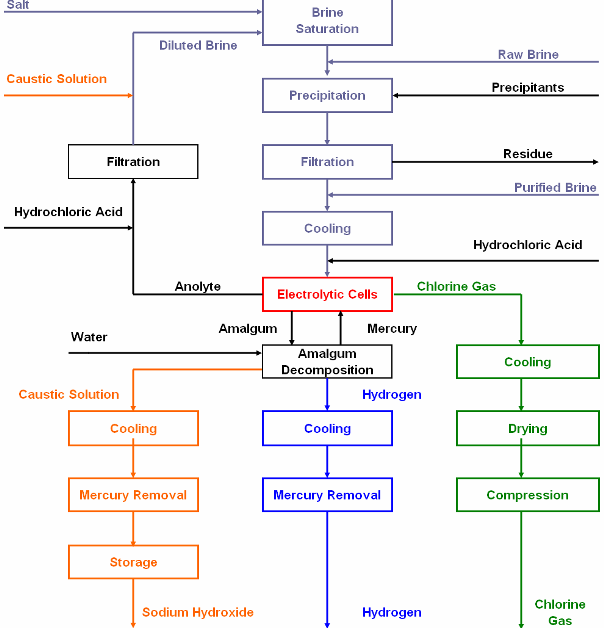
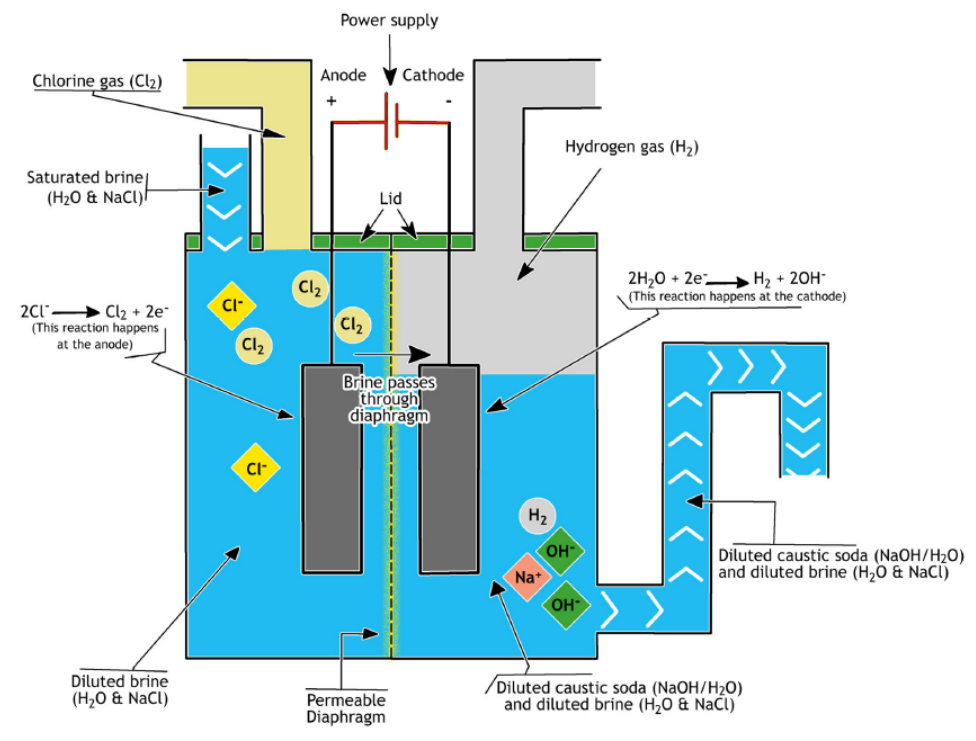
Diaphragm Cell Process
The diaphragm cell process works with the Asbestos separator. Using asbestos helps us separate sodium hydroxide and chlorine, which are the products of the reactions that occur inside this kind of cell.
The production of the diaphragm cell reaction is a liquid named ‘Cell liquor’. This cell liquor is a weak lye that contains 12 to 14 percent of sodium hydroxide (weight percent) and a constant volume of salt.
In order to obtain a 50 percent sodium hydroxide solution, we need to evaporate the resultant lye to increase the concentration of NaOH. With this method, we can separate the extra salt and bring it back to the cycle for reuse.
The caustic soda that is produced with this method has several names such as Diaphragm cell-grade caustic soda, commercial grade, technical grade and technical diaphragm.
In this method, we could have an additional grade of caustic soda named sublime grade. The sublime-grade sodium hydroxide forms as a result of further evaporating the 50% solution and diluting it to reduce the concentration of the salt in the solution.
To increase the concentration of lye from the diaphragm cells, we need a considerable amount of heat to evaporate the water and increase the concentration.
However, these cells could be cheaper than Mercury ones when the steam price is low. They also have less construction prices.
In the below table, we have brought some details of each method.
| More Details of Each Method | |||
|---|---|---|---|
| Item | Mercury | Diaphragm | Membrane |
| Operating Current Density (kA/m2 ) | 8 – 13 | 0.9 – 2.6 | 3 – 5 |
| Cell Voltage (V) | 3.9 – 4.2 | 2.9 – 3.5 | 3.0 – 3.6 |
| NaOH Strength (wt%) | 50 | 12 | 33 – 35 |
| Energy Consumption (kWh/MT Cl2 ) at a Current Density of (kA/m2 ) | 3360 (10) | 2720 (1.7) | 2650 (5) |
| Steam Consumption (kWh/MT Cl2 ) for Concentration to 50% NaOH | 0 | 610 | 180 |
How to Make Solid Caustic Soda?
Solid caustic soda, including flakes and pearls, is produced from liquid caustic soda. Caustic soda flakes are made by the evaporation of caustic soda liquid. Granulated or pearl type is created by fusing liquid caustic soda, a procedure that includes the solidification of liquid caustic soda.








